|
Case Report
Meningo-encephalo-vasculitis, optic neuritis, and thrombotic complications: About a fulminant mucormycosis in a diabetic patient
1 Resident in Radiology, Neuroradiology Department, Specialty Hospital of Rabat, Morocco
2 Resident, Mycology Department, IBN SINA Hospital Center, Rabat, Morocco
3 Professor in Radiology, Neuroradiology Department, Specialty Hospital of Rabat, Morocco
Address correspondence to:
Ibtissam El Ouali
Resident in Radiology, Neuroradiology Department, Specialty Hospital of Rabat, Avenue Lalla Asmaa, AL AZHAR Residency, Salé,
Morocco
Message to Corresponding Author
Article ID: 100020R02IO2022
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
El Ouali I, Hamzaoui A, Diallo ID, Fikri M, Jiddane M, Touarsa F. Meningo-encephalo-vasculitis, optic neuritis, and thrombotic complications: About a fulminant mucormycosis in a diabetic patient. Edorium J Radiol 2022;8(1):1–5.ABSTRACT
Mucormycosis is a destructive, potentially fatal, and opportunistic fungal infection caused by filamentous Mucorales which commonly affect immunocompromised hosts. This infection might take different forms such as gastrointestinal, pulmonary, cutaneous or even a disseminated form, yet the rhinocerebral localization is historically the primary presentation of the disease and most common type. It originates in the nasal mucosa owing to fungal inoculation, then it spreads through paranasal sinuses and orbits to the brain and its vessels especially the cavernous sinus, leading to thrombotic complications including arterial thrombosis. Herein, we present a case of a 35-year-old male with poorly controlled diabetes who presented with decompensated diabetes, in whom the clinical examination finds subtle signs of orbital cellulitis. The patient subsequently had worsening necrotizing orbital cellulitis which required surgical drainage of the left ethmoid along with large spectrum antibiotic therapy; this was complicated by the development of meningo-encephalo-vasculitis as well as cavernous sinus and left internal carotid thrombosis. Tissue cultures revealed evidence of Rhizopus.
Keywords: CT scan, Diabetes, MRI, Mucormycosis
INTRODUCTION
Rhino-orbito-cerebral mucormycosis is the most common form of the disease prevailing in the expanding immunocompromised population.
The management of such infections is a challenging process marked by rapid tissue necrosis as a result of vascular invasion and even patient’s death if the fungal patterns are not identified early.
CASE REPORT
Our patient is a 35-year-old male with poorly controlled diabetes who was hospitalized for decompensated diabetes. Clinical evaluation finds left periorbital edema associated with fever. Despite the antibiotic therapy, his symptoms progressed as he developed periorbital tissue necrosis, confusion, and deterioration of the general status
Complete blood count result was significant for leukocytosis of 13×109 per liter.
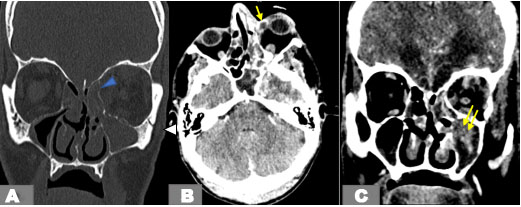
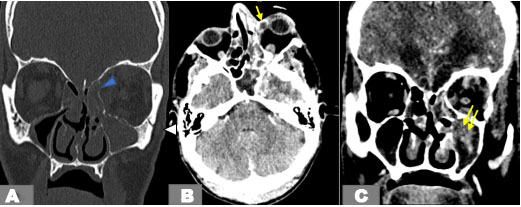
An orbital multislice computed tomography (CT) demonstrated left maxillary and ethmoidal sinusitis with parietal defect, diffuse fat stranding, and soft tissue thickening. The infection spread to the medial orbit and the orbital apex through the nasolacrimal duct (Figure 1).
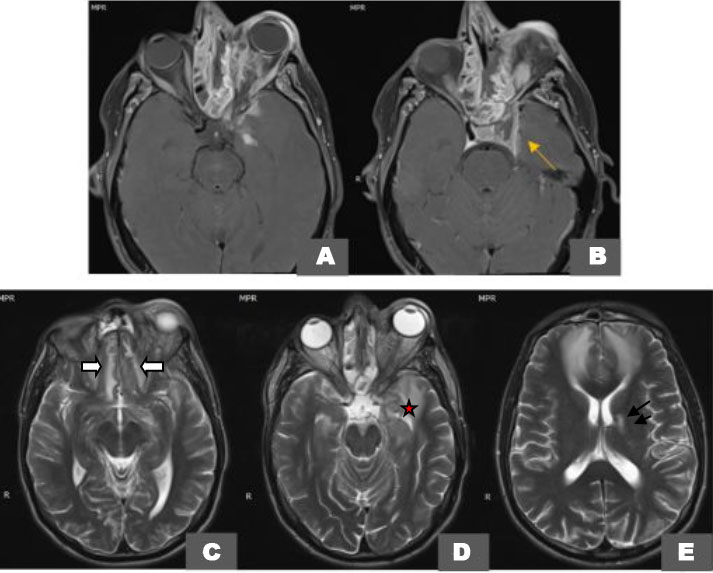
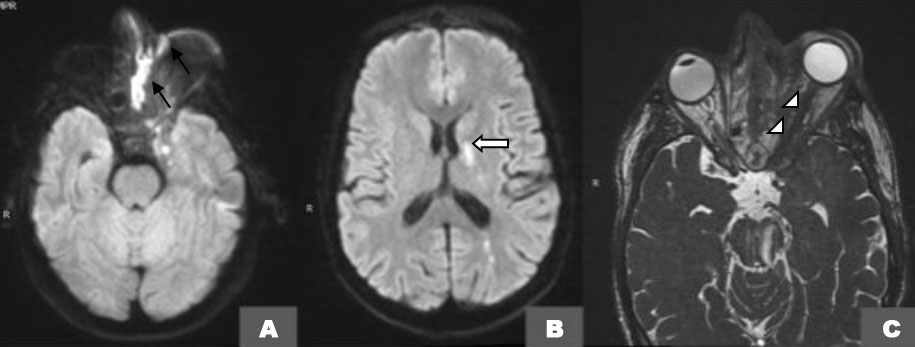
An orbital magnetic resonance imaging (MRI) 1.5 Tesla confirmed the previously described soft tissue and sinus lesions on T1-weighted images (Figure 2A and Figure 2B) and contributed to a better assessment of the extra-ocular muscles and the infectious collection itself especially on T2-weighted fat-saturated axial images (Figure 2C, Figure 2D, Figure 2E) showing a partitioned ill-defined destructive collection of the left maxillary and ethmoid sinuses extending to the orbital apex and orbital gyri resulting in fronto-temporal collections with diffusion restriction (Figure 3A and Figure 3B) encasement of the optic nerve on CISS (Figure 3C), and peripheral enhancement of these collections on post-contrast fat-saturated T1-weighted images (Figure 4A and Figure 4B).
Magnetic resonance angiography (Figure 4C) confirmed the thrombosis of both left cavernous sinus and internal carotid artery responsible of striatocapsular and parietal infarcts seen on the previous sequences.
The patient continued to deteriorate despite aggressive therapy.
A specimen of the biopsy of the necrotic eyelid was sent to the Mycology Laboratory in a sterile tube. Direct examination of a fragment in physiologic water showed multiple hyalin large irregular and non-septate hyphae. This was highly suspicious of a mold. The other fragment was put into culture in three media: sabouraud, sabouraud with chloramphenicol, and sabouraud with chloramphenicol and cycloheximide. The three media were incubated in 27°C and 37°C in two series. Colonies rapidly grew on cultures 72 hours later in all tubes. Microscopic features were characteristics of Mucorales, first white then turning black with cottony texture (Figure 5A).
Cellophane flag method was performed in lactophenol blue solution. It showed sporangiophores arising from root-like rhizoids with numerous spores. This was compatible with the microscopic aspect of Rhizopus sp. (Figure 5B). Parenteral Amphotericin B was then initiated.
DISCUSSION
Mucormycosis is an acute and rapidly progressive fungal disease first described in 1943 by Gregory [1]. The main responsible for human infection are Rhizopus, Mucor, and Absidia.
This opportunistic infection mainly affects immunosuppressed patients, more particularly poorly balanced diabetics [2] about 70% of rhinocerebral mucormycosis cases [3].
Acidosis is the main promoting factor of fungal outbreaks as it increases the level of free iron necessary for their growth. Moreover, studies revealed that Glucose-regulated protein 78 (GRP78) was a putative endothelial cell receptor for rhizopus and other Mucorales necessary for endocytosis and subsequent endothelial cell damage. Interestingly, GRP78 expression involved both iron and glucose [4].
Five clinical forms of mucormycosis are recognized: rhino-orbito-cerebral, gastrointestinal, pulmonary, cutaneous, and disseminated [5].
The rhino-cerebral form is the most common and deadly form. The infection originates in the nasal cavity and then spreads to the paranasal sinuses and medial orbit, to the orbital apex through nasolacrimal duct reaching the brain with spread to the cavernous sinus and internal carotid artery which may result in hemorrhage or thrombosis.
The clinical presentation is not specific and can range from a subtle facial swelling to tissue necrosis with associated fever, headache, reduced visual acuity, and neurological deficit.
Magnetic resonance imaging has a major role in the diagnosis of rhino-cerebral mucormycosis, for early detection of orbital and intracranial complications [6].
Lesions can show variable MR signal characteristics on T1 and T2-weighted images, variable contrast enhancement, and restriction of diffusion. Contrast-enhanced T1-weighted images are helpful in delineating the orbital and intracranial spread [6],[7]. Some findings can be helpful in differentiating mucormycosis from bacterial sinusitis such as the non-enhancement of the involved mucosal tissue on contrast MRI known as the “black turbinate” sign. This phenomenon is indicative of mucosal ischemia secondary to fungal vascular damage. Magnetic resonance spectroscopy has been reported to help differentiate central nervous system (CNS) mucormycosis from bacterial cerebritis, but this modality still needs further validation [7].
Computed tomography scan shows evidence of bony destruction in sinus walls, the turbinate, orbital wall, skull base, and hard palate in 40% of rhino-orbito-cerebral mucormycosis (ROCM) cases and demonstrates almost the same findings as MRI with less sensitivity, such as soft tissue opacification of the sinuses with hyperdense material, nodular mucosal thickening [8].
Imaging discloses the most fearsome complications such as brain infarction and hemorrhage due to diffuse fungal vascular invasion which weakens the walls of blood vessels forming aneurysms and contributes to intravascular thrombi formation. This has been previously described in a study by Lowe and Hudson [9].
We can also find orbital apex syndrome defined as a damage of multiple orbital structures like motor nerves, optic nerve, third cranial nerve paralysis, and proptosis due to venous congestion secondary to cavernous thrombosis, meningitis, brain abscesses, Garcin syndrome mimicking diseases like tuberculosis or neoplasms and finally facial deformity [9].
The differential diagnosis of ROCM includes infectious diseases like tuberculosis, aspergillosis, and other fungal diseases, but also non-infectious inflammatory diseases, such as ocular Sweet’s syndrome, idiopathic orbital inflammatory syndrome, and intraorbital masses, including lymphoma, ocular leukemia, and metastases [4].
Mucormycosis management requires both surgical and medical interventions. Surgical debridement was associated with improved survival in several case series [4]. As for antifungal treatment, amphotericin B, more particularly the liposomal formulation, remains the mainstay therapeutic agent against mucormycosis, but its usage is limited due to nephrotoxicity which makes Posaconazole, a potent and wide-spectrum triazole agent, an attractive alternative for the patient, who is intolerant, or even refractory to amphotericin B [2].
Literature review reported some adjunctive therapeutic modalities like hyperbaric oxygen (HBO) to increase survival rate in diabetic patients (94%) treated with HBO [10].
In our case, the imaging findings were suggestive of an important bony destruction, ethmoido-maxillary abscess, with orbital invasion resulting in class 5 cellulitis with neurological complications manifested in optic neuritis, meningo-encephalo-vasculitis with mycotic thromboembolism.
High clinical-radiological suspicion coupled with early identification is key to the treatment of mucormycosis as it involves a combination of surgical interventions with adjunctive aggressive antifungal therapy [11].
Prognosis is known to be very poor and mortality very high in rhino-cerebral mucormycosis with intracranial extension [12].
CONCLUSION
The purpose behind this report is to foreground the importance of MRI imaging modality in making an early diagnosis and detecting complications, CT scan in detecting any bony defects as complimentary to MRI, and finally culture of specimens allows identification to the genus and species level, and eventually antifungal susceptibility testing.
The spread of mucormycosis due to late diagnosis results in an extensive encephalitis with severe vascular complications; thrombosis of the entire internal carotid artery and cavernous sinus, and clear damage of the orbit, ethmoid, and maxillary sinuses. Such impairment is worth displaying as part of raising awareness about opportunistic infection management in immune suppressed population.
REFERENCES
1.
Skiada A, Pavleas I, Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: An update. J Fungi (Basel) 2020;6(4):265. [CrossRef]
[Pubmed]

2.
Gholinejad Ghadi N, Seifi Z, Shokohi T, et al. Fulminant mucormycosis of maxillary sinuses after dental extraction inpatients with uncontrolled diabetic: Two case reports. [Article in French]. J Mycol Med 2018;28(2):399–402 [CrossRef]
[Pubmed]

3.
Patil A, Mohanty HS, Kumar S, Nandikoor S, Meganathan P. Angioinvasive rhinocerebral mucormycosis with complete unilateral thrombosis of internal carotid artery-case report and review of literature. BJR Case Rep 2016;2(2):20150448. [CrossRef]
[Pubmed]

4.
Chikley A, Ben-Ami R, Kontoyiannis DP. Mucormycosis of the central nervous system. J Fungi (Basel) 2019;5(3):59. [CrossRef]
[Pubmed]

5.
Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clin Infect Dis 2005;41(5):634–53. [CrossRef]
[Pubmed]

6.
Siegal JA, Cacayorinb ED, Nassif AS, et al. Cerebral mucormycosis: Proton MR spectroscopy and MR imaging. Magn Reson Imaging 2000;18(7):915–20. [CrossRef]
[Pubmed]

7.
Han Q, Escott EJ. The black turbinate sign, a potential diagnostic pitfall: Evaluation of the normal enhancement patterns of the nasal turbinates. AJNR Am J Neuroradiol 2019;40(5):855–61. [CrossRef]
[Pubmed]

8.
Cho SJ, Choi YJ, Cho KJ, et al. Image findings in patients with chronic invasive fungal infection of paranasal sinuses. J Neuroradiol 2021;48(5):325–30. [CrossRef]
[Pubmed]

9.
Manjunath KS, Shivaswamy S, Kulkarni JD, Kenkare Venkatachalaiah R. Rhino-orbito-cerebral mucormycosis (ROCM) with internal carotid artery stenosis in a diabetic patient with caries tooth and oroantral fistula. BJR Case Rep 2016;2(2):20150447. [CrossRef]
[Pubmed]

10.
John BV, Chamilos G, Kontoyiannis DP. Hyperbaric oxygen as an adjunctive treatment for zygomycosis. Clin Microbiol Infect 2005;11(7):515–7. [CrossRef]
[Pubmed]

11.
Skiada A, Lass-Floerl C, Klimko N, Ibrahim A, Roilides E, Petrikkos G. Challenges in the diagnosis and treatment of mucormycosis. Med Mycol 2018;56(suppl_1):93–101. [CrossRef]
[Pubmed]

12.
Haliloglu NU, Yesilirmak Z, Erden A, Erden I. Rhinoorbito-cerebral mucormycosis: Report of two cases and review of the literature. Dentomaxillofac Radiol 2008;37(3):161–6. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Acknowledgments
I would like to express my gratitude to my professors and all the colleagues who participated in the completion of this work. The authors declare that they have no competing interests.
Author ContributionsIbtissam El Ouali - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Abdeljalil Hamzaoui - Acquisition of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ibrahima Dokal Diallo - Acquisition of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Meriem Fikri - Conception of the work, Design of the work, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Mohamed Jiddane - Conception of the work, Design of the work, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Firdaous Touarsa - Conception of the work, Design of the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2022 Ibtissam El Ouali et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.